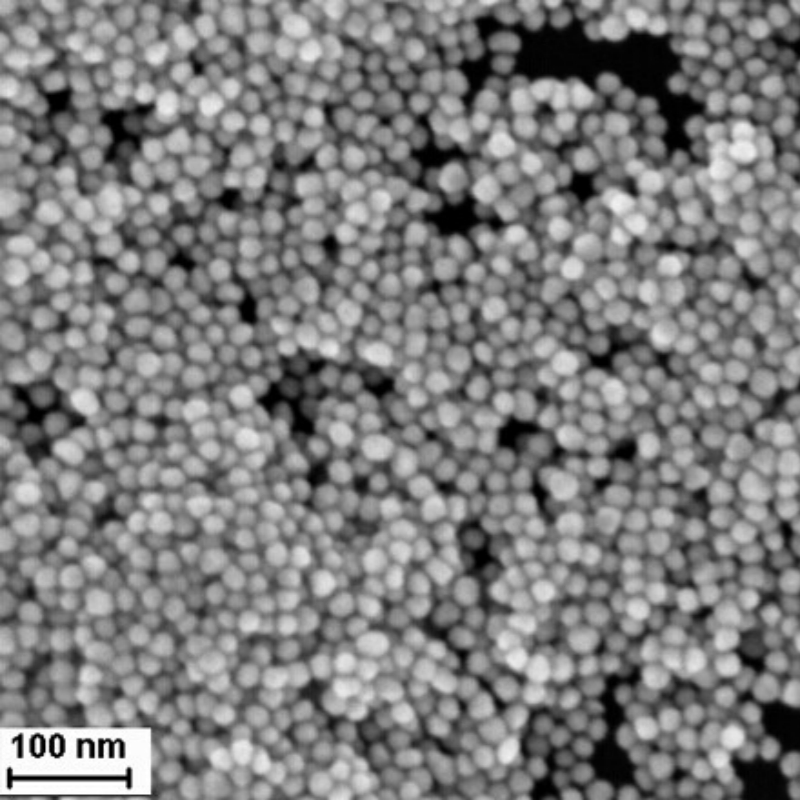
Oil-soluble gold nanoparticles provide optimized hydrophobicity, superior dispersion in organic solvents, and enhanced plasmonic properties. Designed for advanced applications, they ensure efficient integration, extended durability, and high-performance adaptability.
Product Overview
Oil-soluble gold nanoparticles are modified from water-soluble gold nanoparticles by functionalizing with aromatic hydrocarbons to make them dispersible in oil-based solvents. These nanoparticles are able to disperse uniformly in a variety of oil-based solvents, including toluene, xylene, cyclohexane, DMSO, DMF, and others. They exhibit broad application potential in fields such as catalysis, material processing, and optics.
Features
- Stability: Gold nanoparticles maintain good stability in oil-based systems, resisting aggregation or precipitation, and are suitable for long-term storage.
- Dispersion: Capable of uniformly dispersing in oil phases, forming a stable homogeneous system.
- Compatibility: Compatible with a variety of oil-based substances and organic systems, making them suitable for various oily environments.
- Size Effect: The particle size significantly influences the properties and applications of gold nanoparticles, with different sizes offering distinct characteristics.
Applications
- Catalysis: Used as efficient catalysts in organic reactions, enhancing reaction rates and efficiencies.
- Materials Science: Employed in the fabrication of functional nanocomposites, improving the properties of nanocoatings and materials.
- Optics: Due to their unique optical properties, they are useful in the production of specialized optical devices or materials.
- Analytical Chemistry: Can serve as markers or enhancers in analytical detection, improving sensitivity and detection capabilities.
| Technical Parameter | Description |
| Particle Diameter | 5, 10, 15, 20, 40, 60, 80, 100, 120, 160, 180, 200 (customizable as needed) |
| Standard Solvent | Xylene |
| Customizable Solvents | Chloroform, Toluene, Cyclohexane, DMSO, DMF, NMP, Ethyl Acetate, Dichloromethane, Tetraethyl Orthosilicate, etc. |
| Remarks | Concentration is based on water-soluble precursors; after modification with aromatic hydrocarbons to become oil-soluble materials, concentration is difficult to verify. |
 new material
new material